Answer:
Option (C) is correct.
Step-by-step explanation:
Decomposition reaction:
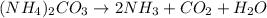
Molar mass of
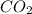 = 44.01 g/mol
= 44.01 g/mol
No. of moles = (mass)/(molar mass)
So, 6.52 g of
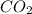 =
=
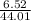 moles of
moles of
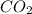 = 0.148 moles of
= 0.148 moles of
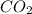
According to balanced equation-
1 mol of
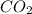 is produced from decomposition of 1 mol of
is produced from decomposition of 1 mol of
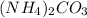
So, 0.148 mol of
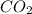 is produced from decomposition of 0.148 mol of
is produced from decomposition of 0.148 mol of
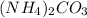
Hence, option (C) is correct.