2.07 M is the molarity of the final solution if 45.0 ml of 6.00M HCl is added to 130 ml of
 O.
O.
Step-by-step explanation:
Data given:
volume of HCl, V1 = 45 ml
molarity of the HCl solution, M1 = 6 M
final volume of the HCl solution after water is added, V2 = 130 ml
final molarity M2 =?
After the dilution, the molarity of the new solution can be found by using the formula:
M1V1 = M2V2
here M1 and V1 are the molarity and volume for concentrated solution, M2 and V2 represents the diluted solution's molarity and volume.
Applying the formula after rearranging the equation, we get
M2 =
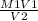
M2 =
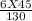
M2 = 2.07 M
The final molarity of the HCl solution is 2.07 M