Answer: pH of the solution after the addition of 600.0 ml of LiOH is 7.
Step-by-step explanation:
The given data is as follows.
Volume of
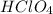 = 900.0 ml = 0.9 L,
= 900.0 ml = 0.9 L,
Molarity of
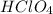 = 0.18 M,
= 0.18 M,
Hence, we will calculate the number of moles of
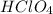 as follows.
as follows.
No. of moles = Molarity × Volume
= 0.18 M × 0.9 L
= 0.162 moles
Volume of NaOH = 600.0 ml = 0.6 L
Molarity of NaOH = 0.27 M
No. of moles of NaOH = Molarity × Volume
= 0.27 M × 0.6 L
= 0.162 moles
This shows that the number of moles of
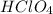 is equal to the number of moles of NaOH.
is equal to the number of moles of NaOH.
Also we know that,

As 1 mole of
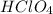 reacts with 1 mole of NaOH then all the hydrogen ions and hydroxide ions will be consumed.
reacts with 1 mole of NaOH then all the hydrogen ions and hydroxide ions will be consumed.
This means that pH = 7.
Thus, we can conclude that pH of the solution after the addition of 600.0 ml of LiOH is 7.