Answer:
Step-by-step explanation:
a).
conc of Ca²⁺ =0.0025 M
pCa = -log(0.0025) = 2.6
logK,= 10.65 So lc = 4.47 x 10.
Formation constant of Ca(EDTA)]-z= 4.47 x 10¹⁰ At pH = 11, the fraction of EDTA that exists Y⁻⁴ is
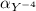 =0.81
=0.81
So the Conditional Formation constant=
 =0.81x 4.47 x10¹⁰
=0.81x 4.47 x10¹⁰
=3.62x10¹⁰
b)
At Equivalence point:
Ca²⁺ forms 1:1 complex with EDTA At equivalence point,
Number of moles of Ca²⁺= Number of moles of EDTA Number of moles of Ca²⁺ = M×V = 0.00250 M × 50.00 mL = 0.125 mol
Number of moles of EDTA= 0.125 mol
Volume of EDTA required = moles/Molarity = 0.125 mol / 0.0050 M = 25.00 mL
V e= 25.00 mL
At equivalence point, all Ca²⁺ is converted to [CaY²⁻] complex. So the concentration of Ca²⁺ is determined by the dissociation of [CaY²⁻] complex.
![[CaY^(2-)] = (Initial,moles,of, Ca^(2+))/(Total,Volume) = (0.125mol)/((50.00+25.00)mL) = 0.001667M](https://img.qammunity.org/2021/formulas/chemistry/college/psd5ag49vfrbipefsyr9eg64x0u0x4xsr4.png)
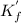
Ca²⁺ + Y⁴ ⇄ CaY²⁻
Initial 0 0 0.001667
change +x +x -x
equilibrium x x 0.001667 - x
![{K^'}_f = ([CaY^(2-)])/([Ca^(2+)][Y^4])=(0.001667-x)/(x.x) =(0.001667-x)/(x^2)\\\\x^2 = \frac{0.001667-x}{{K^'}_f}\\ \\](https://img.qammunity.org/2021/formulas/chemistry/college/es0mnyi12y38upwkw0ty2j1o7w3h8weod3.png)
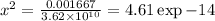
x = 2.15×10⁻⁷
[Ca+2] = 2.15x10⁻⁷ M
pca = —log(2 15x101= 6.7