Here is the correct question
You mix 125 mL of 0.170 M CsOH with 50.0 mL of 0.425 M HF in a coffee-cup calorimeter, and the temperature of both solutions rises from 20.20 °C before mixing to 22.17 °C after the reaction. What is the enthalpy of reaction per mole of ? Assume the densities of the solutions are all 1.00 g/mL, and the specific heat capacities of the solutions are 4.2 J/g · K. Enthalpy of reaction = kJ/mol
Answer:
75.059 kJ/mol
Step-by-step explanation:
The formula for calculating density is:
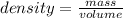
Making mass the subject of the formula; we have :
mass = density × volume
which can be rewritten as:
mass of the solution = density × volume of the solution
= 1.00 g/mL × (125+ 50 ) mL
= 175 g
Specific heat capacity = 4.2 J/g.K
∴ the energy absorbed is = mcΔT
= 175 × 4.2 × (22.17 - 20.00) ° C
= 1594.95 J
= 1.595 J
number of moles of CsOH =
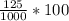
= 0.2125 mole
Therefore; the enthalpy of the reaction =
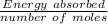
=
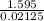
= 75.059 kJ/mol