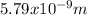Answer:
The uncertainty in the position of the electron is
Step-by-step explanation:
The Heisenberg uncertainty principle is defined as:
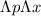 ≥
≥
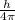 (1)
(1)
Where
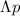 is the uncertainty in momentum,
is the uncertainty in momentum,
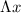 is the uncertainty in position and h is the Planck's constant.
is the uncertainty in position and h is the Planck's constant.
The momentum is defined as:
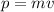 (2)
(2)
Therefore, equation 2 can be replaced in equation 1
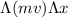 ≥
≥
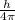
Since, the mass of the electron is constant, v will be the one with an associated uncertainty.
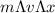 ≥
≥
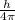 (3)
(3)
Then,
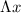 can be isolated from equation 3
can be isolated from equation 3
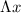 ≥
≥
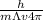 (4)
(4)

But
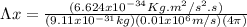
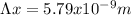
Hence, the uncertainty in the position of the electron is