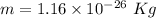Answer:
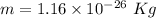
Step-by-step explanation:
Given,
Magnetic field, B = 0.5 T
Electric field, E = 1.2 x 10⁵ V/m
strength of the magnetic field that separates the ions, Bo=0.750 T
Radius, r = 2.32 cm
Relation of charge to mass ratio is given by
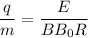
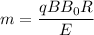
Substituting all the values
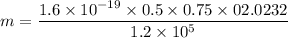
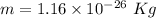
Mass of Li ions is equal to