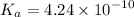Answer:
Step-by-step explanation:
Write the base reaction of NaA with water:
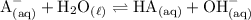
Hence, the equilibrium constant expression for the reaction is:
![\displaystyle K_b = \frac{[\text{OH}^-][\text{HA}]}{[\text{A}^-]}](https://img.qammunity.org/qa-images/2023/formulas/chemistry/college/6dk19q87l720vz2g1az8.png)
Thus, to find Ka, we can find Kb and use the fact that Ka × Kb = Kw.
From the reaction and initial concentration of NaA, create an ICE chart:
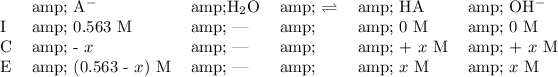
Find [OH⁻] from the given pH:
![\displaystyle \begin{aligned} \text{pH} +\text{pOH} & = 14.00 \\ \\ \text{pOH} & = 14.00 - \text{pH} \\ \\ & = 14.00 - (11.56) \\ \\ & = 2.44 \\ \\ -\log[\text{OH}^-] & = 2.44 \\ \\ [\text{OH}^-] &= 10^(-2.44) \\ \\ & =0.00363 \text{ M}= 3.63* 10^(-3) \text{ M} = x\text{ M}\end{aligned}](https://img.qammunity.org/qa-images/2023/formulas/chemistry/college/if407t8zfawcut5mdg86.png)
Solve for all species concentrations at equilibrium from the found x value:
![\displaystyle [\text{HA}] = [\text{OH}^-] = 3.63* 10^(-3) \text{ M}](https://img.qammunity.org/qa-images/2023/formulas/chemistry/college/qw6khflwt7knvptive95.png)
And:
![\displaystyle \begin{aligned} \ [\text{A}^-] & = 0.563 - 3.63* 10^(-3) \text{ M}\\ \\ & = 0.559\text{ M}\end{aligned}](https://img.qammunity.org/qa-images/2023/formulas/chemistry/college/z6ykmbgkn5etbe1iu4pd.png)
Find Kb:
![\displaystyle \begin{aligned} \displaystyle K_b &= \frac{[\text{OH}^-][\text{HA}]}{[\text{A}^-]} \\ \\ & = ((3.63* 10^(-3))(3.63* 10^(-3)))/((0.559))\\ \\ & = 2.36* 10^(-5)\end{aligned}](https://img.qammunity.org/qa-images/2023/formulas/chemistry/college/dhojl1v9njwzav8qkgvb.png)
Find Ka:
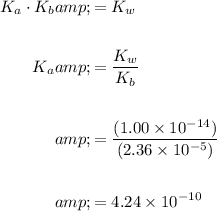
In conclusion: