Answer:
3.71%
Step-by-step explanation:
The phenolphthalein endpoint refers to the reactions:
OH⁻ + H⁺ → H₂O
CO₃⁻² + H⁺ → HCO₃⁻
While the methyl orange endpoint to:
HCO₃⁻ + H⁺ → H₂CO₃
So the additional volume required for the second endpoint tells us the amount of HCO₃⁻ species, which in turn is the total amount of Na₂CO₃ in the sample:
0.700 mL * 0.100 M *
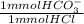 = 0.07 mmol HCO₃⁻
= 0.07 mmol HCO₃⁻
Now we calculate the mass of Na₂CO₃, using its molecular weight:
0.07 mmol HCO₃⁻ = 0.07 mmol Na₂CO₃
0.07 mmol Na₂CO₃ * 106 mg/mmol = 7.42 mg Na₂CO₃
No calculations using the volume of the first equivalence point are required because the problem already tells us the mass of the sample is 0.200 g.
0.200 g ⇒ 0.200 * 1000 = 200 mg
%Na₂CO₃ = 7.42 mg/200 mg * 100 = 3.71%