Answer:
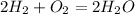
Step-by-step explanation:
The chemical formula for hydrogen is H while chemical formula for oxygen is O
Considering that two moles of hydrogen and 2 moles of oxygen react to form water, the equation will be
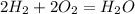
The number of oxygen molecules don't balance on both LHS and RHS hence to balance it, we put it as follows
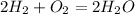
Normally, two moles of hydrogen require only one mole of oxygen to form two moles of water.