6.81% is the percent yield when 12 g of CO2 are formed experimentally from the reaction of 0.5 moles of C8H18 reacting with excess oxygen.
Step-by-step explanation:
Data given:
mass of carbon dioxide formed = 12 grams (actual yield)
atomic mass of CO2 = 44.01 grams/mole
moles of
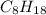 = 0.5
= 0.5
Balanced chemical reaction:
2
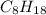 + 25
+ 25
 ⇒ 16 C
⇒ 16 C
 + 18
+ 18
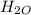
number of moles of carbon dioxide given is
number of moles =

number of moles=
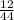
number of moles of carbon dioxide gas = 0.27 moles
from the reaction 2 moles of
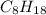 reacts to produce 16 moles of C
reacts to produce 16 moles of C

So, when 0.5 moles reacted it produces x moles
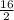 =
=
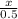
x = 4
4 moles of carbon dioxide formed, mass from it will give theoretical yield.
mass = number of moles x molar mass
mass = 4 x 44.01
= 176.04 grams
percent yield =
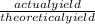 x 100
x 100
percent yield =
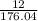 x 100
x 100
percent yield = 6.81 %