Answer:
Check the explanation
Step-by-step explanation:
Acidipic Acid (which is an essential dicarboxylic acid for manufacturing purposes with about 2. 5 billion kilograms produced per year. It is mainly used for the production of nylon and its related materials.)
Going by the question, since the
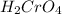 is comparatively mild oxidizing agent than the
is comparatively mild oxidizing agent than the
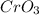 , it only oxidizes the carbon group
, it only oxidizes the carbon group
Kindly check the attached image below for the full explanation to the question above.