Complete Question
Identify the orbitals that overlap to form the C−Cl bonds in CH2Cl2.
a. carbon sp3 hybrid orbital with a singly occupied chlorine 3s orbital
b. carbon sp2 hybrid orbital with a singly occupied chlorine 3p orbital
c. carbon sp3 hybrid orbital with chlorine sp3 hybrid orbital
d. carbon sp3 hybrid orbital with a singly occupied chlorine 3p orbital
e. a singly occupied carbon 2p orbital with chlorine sp3 hybrid orbital
Answer:
Correct Option is D
Step-by-step explanation:
The molecular formula of the compound given is
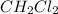
The structural formula for this given compound is shown on the first uploaded image
Looking at the structural formula we see that all the bonds are single bonds which shows that carbon is sp3 hybridzied which means that one 2s orbital of carbon has mixed with 3 2p orbital to for a form a four hybrid orbital as shown on the second uploaded image
For the clorine the outer shell is containing two 3s orbital which are completely filled and a 6 3p orbital which requires an electron to complete it as shown on the uploaed image
Hence the bond between the carbon and the clorine is between a
sp3 hybridzied orbital and a 3p orbital
Note: each orbital contains a single electron
hhhhhhkkkkkkk