The molarity of the acid is 1.25 M.
Step-by-step explanation:
We have to find the molarity of HCl by writing the balanced equation that is the neutralization reaction of Hydrochloric acid with the base, Sodium Hydroxide, NaOH.
HCl + NaOH → NaCl + H₂O
From the balanced equation, we can find that one mole of an acid is used to neutralize one mole of a base.
As per the law of volumetric analysis, we can write the equation as,
V1M1 = V2M2
Here V1 and M1 being the volume and molarity of the NaOH
V2 and M2 being the volume and molarity of HCl
V1 = 250 ml
M1 = 0.50 M
V2 = 100 ml
M2 = ?
Now the above equation can be rearranged to find the molarity of the acid as,
M2 =
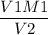
=
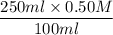
= 1.25 M
So the molarity of the acid is 1.25 M.