Answer:
When the volume is 60 cubic centimeters, the pressure is 4.26 pounds
Step-by-step explanation:
Let V be the volume of the gas and P be its pressure.
The volume of a gas in a container varies inversely with the pressure on the gas:
V = k/P
VP = k, where k represents constant temperature
Given the volume is 32 cubic centimeters at a pressure of 8 pounds
where V1 = 32, P1 = 8 ... (1)
The pressure when the volume is 60 cubic centimeters,
V2 = 60, P2 = ? ... (2)
Comparing eq (1) and (2)
V1 P1 = V2 P2
32 (8) = 60 (P2)
256 = 60 (P2)
P2 =
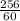
P2 = 4.26 pounds