1.02 moles of the gas is present in the sample.
The conditions in which the gases deviate from ideal behavior is high pressure and low temperatures.
Correct option is 2.
Step-by-step explanation:
Data given:
volume of the gas = 31.2 litres
temperature of the gas = 28 degrees or 301.15 K
pressure of the gas = 82.6 kPa or 0.815 atm
R (Gas constant) = 0.0820 Latm/moles K
number of moles =?
From the ideal gas law, we have
PV = nRT
rearranging the equation:
n =
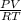
n =
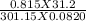
n = 1.02 moles
At high pressure and low temperature an ideal gas deviates from ideal behaviour. Under high pressure the gas molecules get closer to each other and intermolecular force acts on them as molecules attract each other while in ideal case gas has no attractions in its molecules.