Volume of the nitrogen gas = 49.8 L
Step-by-step explanation:
It is given that the pressure, number of moles and temperature of nitrogen gas, and gas constant value being constant and it is taken as 0.08206 L atm mol⁻¹K⁻¹.
Temperature = T = 75°C = 75 + 273 = 348 K
Pressure = P = 0.992 atm
Number of moles = n = 1.73 moles
We have to use the ideal gas equation, PV = nRT, and rearranging the equation to get Volume in litres.
V =
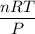
=
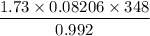
= 49.8 L
So the volume of Nitrogen gas = 49.8 L