Answer:
Therefore the volume of the gas is 32 cubic feet.
Explanation:
Given that,
The volume of a gas varies directly with its temperature and inversely with the pressure.
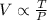
V= Volume of the gas at constant mass
T= Temperature at constant mass
P= pressure at constant mass
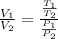
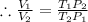 .
.
Given that,
At constant mass, the volume of a certain gas is 15 cubic feet and a pressure of 40 pound per square inch at a temperature 300 k.
 =15 cubic feet,
=15 cubic feet,
 =40 pound per square inch and
=40 pound per square inch and
 = 300 K
= 300 K
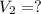 ,
,
 =20 pound per square inch and
=20 pound per square inch and
 = 320k
= 320k
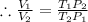 .
.
Plugging all values
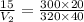
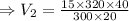
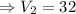 cubic feet
cubic feet
Therefore the volume of the gas is 32 cubic feet.