Answer:
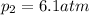
Step-by-step explanation:
Hello,
In this case, when the pressure-temperature-volume behavior is analyzed in ideal gases, the following equation is employed:

Whereas the subscript 1 accounts for the initial state and the subscript 2 for the final state. Therefore, solving for the final pressure we obtain:
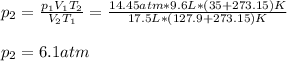
Best regards.