A gas system which has a volume of 250 ml at 280K. If the pressure keep constant, 223.15 ml is the volume of the gas system at 250 K.
Step-by-step explanation:
Data given:
A gas system given with conditions as:
initial volume of gas, V1= 250 ml
initial temperature of the gas, T1= 280 K
Pressure = constant
Final volume of the gas V2= ?
Final temperature of the gas, T2 = 250 K
From the Charles' Law
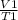 =
=
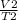
rearranging the equation, we get
V2 =
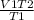
Putting the values in the equation, we get
V2 =
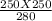
V2 = 223.21 ml
When the gas system has its temperature reduced to 250 K the volume is reduced to 223.21 ml.