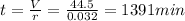Answer:
1391 minutes
Step-by-step explanation:
To solve the problem, we need to find first the volume of gas present in the room.
We can do it by using the equation of state for an ideal gas:
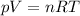
where
p = 1 atm is the pressure
V is the volume
n = 1 mol is the number of moles (not given in the problem, so we assume its value)
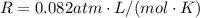 is the gas constant
is the gas constant
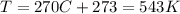 is the temperature
is the temperature
Solving for V,
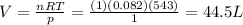
So, there are 44.5 L of gas in the room.
The gas can be removed at a rate of
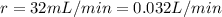
Therefore, the time it takes is: