0.922 atm is the total pressure of gas mixture that contains oxygen, nitrogen and helium gases.
Step-by-step explanation:
Data given:
partial pressure of the oxygen p
 = 0.197 atm
= 0.197 atm
partial pressure of the nitrogen gas p
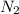 = 0.461 atm
= 0.461 atm
partial pressure of the helium gas pHe = 0.264
total pressure P in the gas mixture= ?
We know that individual gases exert pressure on the mixture of gases independently. From Dalton's law of pressure it is said that sum of partial pressure of all the gases in the mixture equals its total pressure. So,
Ptotal = p
 + p
+ p
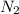 + pHe
+ pHe
putting the values in the mixture:
Ptotal = 0.197 + 0.461+ 0.264
Ptotal = 0.922 atm
Total pressure is 0.922 atm.