Answer:
The Ksp (PbCl₂) of dissolve PbCl₂ is 6.431×10⁻⁵ (mol/L)³
Step-by-step explanation:
PbCl₂ (s) ⇒ Pb²⁺ (aq) + 2Cl⁻ (aq)
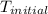 = a - -
= a - -
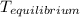 = a - s s 2s
= a - s s 2s
T=time , a=initial condition , s=solubility on concentration
Con. [Pb²⁺(aq)] = 0.0159 M
Con. [Cl⁻(aq)] = 0.0318 M
Ksp = [Pb²⁺] [Cl⁻]²
= (S) (2S)²
= (0.0159 M) (2 × 0.0318)²
Ksp (PbCl₂) = 6.431×10⁻⁵ M³