Answer : The concentration of
 (g) in parts per million is, 8 ppm
(g) in parts per million is, 8 ppm
Explanation : Given,
Mass of oxygen gas (solute) = 0.008 g
Mass of water (solvent) = 1000 g
First we have to calculate the mass of solution.
Mass of solution = Mass of solute + Mass of solvent = 0.008 + 1000 = 1000.008 g
Now we have to calculate the concentration of
 (g) in parts per million.
(g) in parts per million.
ppm : It is defined as the mass of solute present in one million
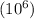 parts by mass of the solution.
parts by mass of the solution.
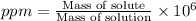
Now put all the given values in this expression, we get
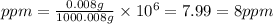
Therefore, the concentration of
 (g) in parts per million is, 8 ppm
(g) in parts per million is, 8 ppm