This is an incomplete question, here is a complete question.
What is the pH of a solution of 0.20 M HNO₂ containing 0.10 M NaNO₂ at 25°C, given Ka of HNO₂ is 4.5 × 10⁻⁴?
Answer : The pH of the solution is, 3.05
Explanation : Given,
 for HNO₂ = 4.5 × 10⁻⁴
for HNO₂ = 4.5 × 10⁻⁴
Concentration of
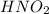 = 0.20 M
= 0.20 M
Concentration of
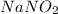 = 0.10 M
= 0.10 M
First we have to calculate the value of
 .
.
The expression used for the calculation of
 is,
is,
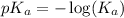
Now put the value of
 in this expression, we get:
in this expression, we get:
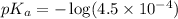
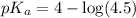
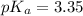
Now we have to calculate the pH of the solution.
Using Henderson Hesselbach equation :
![pH=pK_a+\log ([Salt])/([Acid])](https://img.qammunity.org/2021/formulas/biology/college/z944fnahhldpjolfrvealc6q9baj5h69q3.png)
![pH=pK_a+\log ([NaNO_2])/([HNO_2])](https://img.qammunity.org/2021/formulas/chemistry/high-school/4exq7kvj7jc7wp3gxx4koq7emwby6cblpj.png)
Now put all the given values in this expression, we get:
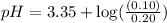
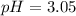
Therefore, the pH of the solution is, 3.05