Answer:
No
Step-by-step explanation:
Molarity is calculated as:
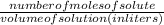
No of mole of substance is calculated as:
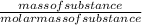
From the formula for calculating the number of mole, the more the mass of a substance, the more the number of moles.
Also, from the formula for calculating the molarity of a solution, the more the number of moles, the more the molarity.
Therefore, if the Na2CO3 precipitate was not dry before the mass was taken, it will result in a higher number of mole (more mass due to presence of water) and hence, a higher molarity.
The molarity calculated should have been too high instead of too low.