Answer: Mass of water required is 1268.18 g.
Step-by-step explanation:
It is mentioned that the energy intake of 0.489 pounds of cheese is 3100 kJ.
And, 1 mole of water takes 44.0 kJ of energy. Hence, we will calculate the molar mass of water as follows.
Molar mass of is =
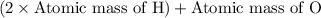
=
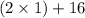
= 18 g/mol
This means that 18 g of water takes 44.0 kJ of energy
. Therefore, mass of water required for 3100 kJ of energy will be calculated as follows.
Mass =
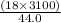
= 1268.18 g of water
Thus, we can conclude that mass of water required is 1268.18 g.