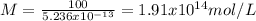Answer:
a) The volume is 5.236x10⁻¹³L
b) The molarity of a single virus is 1.91x10¹² mol/L
c) The molarity for a 100 virus particles is 1.91x10¹⁴ mol/L
Step-by-step explanation:
a) Given:
D = diameter of the cell = 10 μm
r = radius = 10/2 = 5 μm
The volume of the spherical cell is equal:
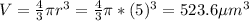
If 1 μm³ = 1x10⁻¹⁵L, then 523.6 μm³ = 5.236x10⁻¹³L
b) The molarity is:
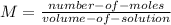
For a single virus within the cell
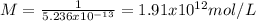
c) For a 100 virus particles the molarity is: