Answer:
Average molar bond enthalpy of C-H bond in
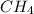 is 415.825 kJ/mol.
is 415.825 kJ/mol.
Step-by-step explanation:
Reaction:
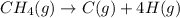
Heat of reaction (energy needed to break 4 moles of C-H bond in 1 mol of
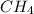 ) =
) =
![\Delta H^(0)=[1mol* \Delta H_(f)^(0)(C)_(g)]+[4mol* \Delta H_(f)^(0)(H)_(g)]-[1mol* \Delta H_(f)^(0)(CH_(4))_(g)]](https://img.qammunity.org/2021/formulas/chemistry/high-school/qi672z5qwzz9bg30w8mtdzx7dtvk2pgiiw.png)
=
![[1mol* 716.7(kJ)/(mol)]+[4mol* 218.0(kJ)/(mol)]-[1mol* -74.6(kJ)/(mol)]=1663.3kJ](https://img.qammunity.org/2021/formulas/chemistry/high-school/yhlmsd7avh9laga5s8u2xmc39v2endi73z.png)
1 mol of
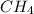 contain 4 moles of C-H bonds.
contain 4 moles of C-H bonds.
So, average molar bond enthalpy of C-H bond in
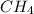
=
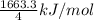
= 415.825 kJ/mol