Answer:
The total pressure P = 1.642 atm
Step-by-step explanation:
From the dalton's law
Total pressure of the mixture is
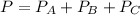 ----- (1)
----- (1)
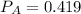 atm
atm
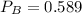 atm
atm
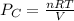
n = 0.24 mole
R =
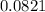
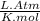
T = 41 °c = 314 K
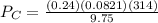
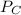 = 0.634 atm
= 0.634 atm
From equation (1)
P = 0.419 + 0.589 + 0.634
P = 1.642 atm
Thus the total pressure P = 1.642 atm