Answer: 438 K
Step-by-step explanation:
According to Gibbs equation:
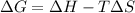
 = Gibb's free energy change
= Gibb's free energy change
 = enthalpy change = 20.1 kJ/mol = 20100 J/mol
= enthalpy change = 20.1 kJ/mol = 20100 J/mol
T = temperature
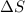 = entropy change = 45.9 J/Kmol
= entropy change = 45.9 J/Kmol
A reaction is at equilibrium when
 = Gibb's free energy change is zero and becomes spontaneous when
= Gibb's free energy change is zero and becomes spontaneous when
 = Gibb's free energy change is negative.
= Gibb's free energy change is negative.
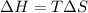
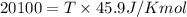
T=437.9K
Thus the temperature at which the reaction change from nonspontaneous to spontaneous in the forward direction is 438 K