25 ml of the 12M solution will be diluted with water till volume made upto 100 ml to get a solution of 3M sulphuric acid.
Step-by-step explanation:
Data given:
stock solution molarity (initial) = 12 M
Required molarity (final)= 3M
Required volume (final)= 100ml
final volume = ?
So the formula used for dilution is:
Initial molarity X Initial volume = final molarity x final volume
initial volume =
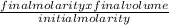
putting the given values in the equation:
initial volume =
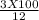
= 25 ml
so, 25 ml of the original solution that is of 12 M will be diluted with water and volume made to 100ml will give a solution of 3M.