Answer:
This reaction is a displacement reaction.
Step-by-step explanation:
Both elements and compounds are chemical substances. However, there's only one type of atom in an element. In a compound, there are at least two different kinds of atoms.
For example,
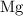
 is an element. Magnesium is the only type of atom that it contains.
is an element. Magnesium is the only type of atom that it contains.
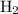 is also an element. Hydrogen is the only type of atom that it contains (Even though there are two hydrogen atoms in each
is also an element. Hydrogen is the only type of atom that it contains (Even though there are two hydrogen atoms in each
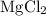 is a compound. It contains both magnesium atoms and chlorine atoms.
is a compound. It contains both magnesium atoms and chlorine atoms.
In a displacement reaction, an element reacts with a compound to produce another element and a different compound. In this reaction,
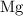 (an element) reacts with
(an element) reacts with
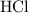 (a compound)
(a compound) - to produce
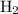 (another element) and
(another element) and
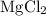 (another compound).
(another compound).
Hence this reaction is a displacement reaction.
As a side note, this reaction matches the pattern:
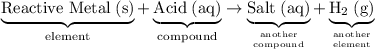 .
.
In other words, if the
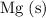 here is replaced with a different metal that is sufficiently reactive (such as aluminum
here is replaced with a different metal that is sufficiently reactive (such as aluminum
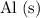 ,) the reaction should still be a displacement reaction.
,) the reaction should still be a displacement reaction.