Answer with Explanation:
Density of copper(Cu)=
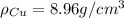
Density of zinc(Zn)=
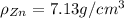
Atomic mass of copper=
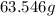
Atomic mass of zinc=65.38 g
We know that
Volume=
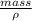
Using the formula
Volume of copper=
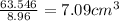
Volume of zinc=
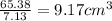
Volume is inversely proportional to the density.
Pre -1982 penny made by copper and post-1982 penny made by zinc.
Therefore, volume of post-1982 penny is greater than the volume of pre-1982 penny because density of zinc is less than the density of copper.