Answer:
46 degrees C
Step-by-step explanation:
We use Charles' Law:
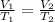 , where V is the volume and T is the temperature in Kelvin.
, where V is the volume and T is the temperature in Kelvin.
Here, V_1 = 1.16 L and T_1 = 23 + 273 = 296 K. Also, V_2 = 1.25 L. Plugging these into the equation, we have:
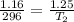
Cross-multiplying, we have:
1.16
 = 1.25 * 296 = 370 ⇒
= 1.25 * 296 = 370 ⇒
 = 370/1.16 ≈ 319 K
= 370/1.16 ≈ 319 K
We want to convert this back to Celsius, so we just subtract 273 from 319:
319 - 273 = 46 degrees Celsius.
Hope this helps!