There are 4.12 × 10²⁴ particles present in the 400 grams of sodium chloride.
Step-by-step explanation:
We need to find the number of particles present in given mass of sodium chloride.
For that we have to convert the given mass into moles by dividing it with its molar mass, so that it can get converted.
Moles =
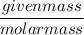
=
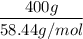
= 6.84 moles
Now this moles is converted into particles by multiplying it with the Avogadro's number of particles as,
Particles present = 6.84 × 6.022 × 10²³
= 4.12 × 10²⁴ particles
So there are 4.12 × 10²⁴ particles present in the given mass of sodium chloride.