Answer:
17.6kJ
Step-by-step explanation:
The mass of ethanol given is 161.g
Now we first find the number of moles from this.
Number of moles =

Molar mass of C₂H₅OH = 2(12) + 5(1) + 16 + 1 = 46g/mol
Number of moles of ethanol =
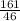 = 3.5mol
= 3.5mol
Since the heat of fusion is 5.02kJ/mol;
Heat of fusion is a measure of the amount of energy needed to melt a substance.
Amount of energy needed to melt = 3.5mol x 5.02kJ/mol
= 17.6kJ