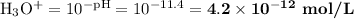Answer:
[H₃O⁺] = 4.3 × 10⁻¹² mol·L⁻¹; [OH⁻] = 2.4 × 10⁻³ mol·L⁻¹;
pH = 11.4; pOH = 2.6
Step-by-step explanation:
The chemical equation is
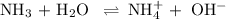
For simplicity, let's re-write this as
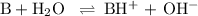
1. Calculate [OH]⁻
(a) Set up an ICE table.
B + H₂O ⇌ BH⁺ + OH⁻
0.310 0 0
-x +x +x
0.310-x x x
![K_{\text{b}} = \frac{\text{[BH}^(+)]\text{[OH}^(-)]}{\text{[B]}} = 1.8 * 10^(-5)\\\\(x^(2))/(0.100 - x) = 1.8 * 10^(-5)](https://img.qammunity.org/2021/formulas/chemistry/college/fvxhqf4mms18rd3028ojx3deguagq0rxcx.png)
Check for negligibility:
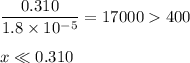
(b) Solve for [OH⁻]
![(x^(2))/(0.310) = 1.8 * 10^(-5)\\\\x^(2) = 0.310 * 1.8 * 10^(-5)\\x^(2) = 5.58 * 10^(-6)\\x = \sqrt{5.58 * 10^(-6)}\\x = \text{[OH]}^(-) = \mathbf{2.4 * 10^(-3)} \textbf{ mol/L}](https://img.qammunity.org/2021/formulas/chemistry/college/tfvuwruahgn8vpf720url1tkf0svqflz0e.png)
2. Calculate the pOH
![\text{pOH} = -\log \text{[OH}^(-)] = -\log(2.4 * 10^(-3)) = \mathbf{2.6}](https://img.qammunity.org/2021/formulas/chemistry/college/gizfpns3x0zfcqzy40gkmoa6qenuxlc8yd.png)
3. Calculate the pH
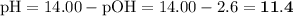
4 Calculate [H₃O⁺]