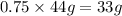Answer:
mass of CO_2 formed=
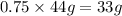
Step-by-step explanation:
First balance the chemical reaction:
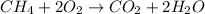
molecular mass of
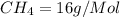
molecular mass of
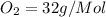
mass given of
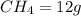
mole of
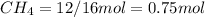
mass given of
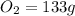
mole of
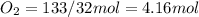
from the above balanced equation:
1 mole of
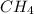 need 2 mole of
need 2 mole of
 gas
gas
therefore 0.75 mole of
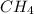 will react with 1.5 mole of
will react with 1.5 mole of
 only
only
but we have 4.16 mole
 gas
gas
hence
 will be the excess reagent and
will be the excess reagent and
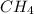 will be the limiting eagent so production of
will be the limiting eagent so production of
 will depenf on the quantity of limiing reagent i.e.
will depenf on the quantity of limiing reagent i.e.
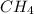
from the above balanced equation:
1 mole of
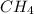 gives 1 mole of
gives 1 mole of
 on complete reacion with
on complete reacion with

hence,
0.75 mole of
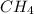 gives 0.75 mole of
gives 0.75 mole of
 on complete reacion with
on complete reacion with

mass of CO_2 formed=