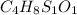Answer:
The empirical formula of the compound =
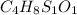
Step-by-step explanation:
Mass of carbon dioxide gas = 59.060 mg = 0.059060 g
1 mg = 0.001 g
Moles of carbon dioxide =
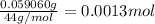
Moles of carbon in 0.0013 moles of carbon dioxide gas = 1 × 0.0013 mol = 0.0013 mol
Mass of 0.0013 moles of carbon =
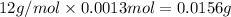
Mass of water = 24.176 mg = 0.024176
Moles of water =
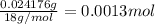
Moles of hydrogen in 0.0013 moles of water = 2 × 0.0013 mol = 0.0026 mol
Mass of 0.0013 moles of hydrogen=
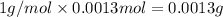
Mass of sulfur dioxide = 20.326 mg = 0.020326 g
Moles of sulfur dioxide =
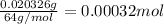
Moles of sulfur in 0.00032 moles of sulfur dioxide = 1 × 0.00032 mol = 0.00032 mol
Mass of 0.00032 moles of sulfur =
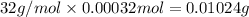
Mass of oxygen in the sample = x
Mass of sample = 33.153 mg = 0.033153 g
0.033153 g = 0.0156 g + 0.0013 g + 0.01024 g + x
x = 0.006013 g
Moles of oxygen =
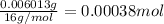
For empirical formula divide the lowest number of moles of elemnt from all the moles of the all the elements:
Carbon :
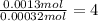
Hydrogen:
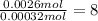
Sulfur :
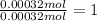
Oxygen :
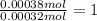
The empirical formula of the compound =