Answer:
Step-by-step explanation:
Some data are garbled, but they seem to be 1 liter of 1M H₂SO₄. Based on that, the solution is as follows:
1. Data:
a) mole ratio: 1 mol H₂SO₄ : 2 mol NaOH
b) NaOH:
i) V = ?
ii) M = 1 M
c) H₂SO₄ :
i) V = 1 liter
ii) M = 1 M
2) Solution
a) Determine the number of moles of H₂SO₄
Use the molarity (M) formula:
- M = n/V ⇒ n = M × V = 1M × 1 liter = 1 mol H₂SO₄
b) Determine the number of moles of NaOH
Set a proportion with the mole ratio:
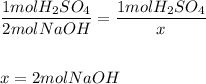
c) Determine the volume of NaOH
Use the molarity formula:
- M = n/V ⇒ V = n/M = 2mol / 1M = 2 liter ← answer