Answer:
It increases by a factor of four.
Step-by-step explanation:
To answer this question, we can use the pressure's law, which states that:
"For a constant mass of an ideal gas kept at constant volume, the pressure of the gas is directly proportional to the absolute temperature of the gas"
Mathematically:
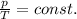
where
p is the pressure of the gas
T is its absolute temperature
The equation can be rewritten as
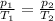
In this problem we have:
 , since the absolute temperature of the gas is increased by 4 times
, since the absolute temperature of the gas is increased by 4 times
Here we want to find p2; solving for it, we find:
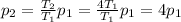
So, the pressure
It increases by a factor of four.