Methane gas and chlorine gas react to form hydrogen chloride gas and carbon tetrachloride gas. What volume of hydrogen chloride would be produced by this reaction if 3.16 L of chlorine were consumed at STP.
Be sure your answer has the correct number of significant digits.
Answer: Thus volume of carbon tetrachloride that would be produced is 0.788 L
Step-by-step explanation:
According to ideal gas equation:

P = pressure of gas = 1 atm (at STP)
V = Volume of gas = 3.16 L
n = number of moles = ?
R = gas constant =
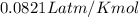
T =temperature =
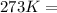
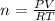
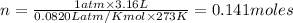
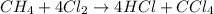
According to stoichiometry:
4 moles of chlorine produces = 1 mole of carbon tetrachloride
Thus 0.141 moles of methane produces =
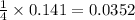 moles of carbon tetrachloride
moles of carbon tetrachloride
volume of carbon tetrachloride =
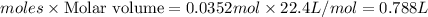
Thus volume of carbon tetrachloride that would be produced is 0.788 L