Answer:
Stoichiometric coefficient of hydrogen gas is 1.
Stoichiometric coefficient of palmitic acid is 1.
Step-by-step explanation:
Addition of hydrogen to double bond is termed as hydrogenation reaction.
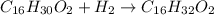
According to stoichiometry, 1 mole of palmitoleic acid reacts with 1 mole of hydrogen gas to give 1 mole of palmitic acid.
Stoichiometric coefficient of hydrogen gas is 1.
Stoichiometric coefficient of palmitic acid is 1.