Answer:
About 3.5 kJ.
Step-by-step explanation:
Recall the heat transfer equation:
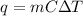
Where q is the heat; m is the mass of the substance; C is its specific heat; and ΔT is the change in temperature.
The given specific heat of iron is 462 J/kg -°C.
Substitute and evaluate. Ensure correct units:
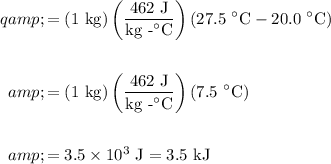
Therefore, the block of iron absorbed about 3.5 kJ of heat.