Answer:
 atoms NaOH
atoms NaOH
Step-by-step explanation:
To figure this out, we need to use Avogadro's number, which is
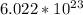 . This number denotes the number of atoms in 1 mole of any substance.
. This number denotes the number of atoms in 1 mole of any substance.
Right now, we have 1.25 moles of NaOH. So, let's set up an equation:
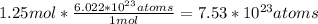
Thus, the answer is
 atoms NaOH.
atoms NaOH.
Hope this helps!