Answer:
- Question 1: You cannot make sure the ballon will not burst before it reaches 3,000m altitude because the volume will exceed 47.0 liters
Step-by-step explanation:
1. Data
- Maximum volume of the balloon: 47.0 liters
At 3,000 m:
- Temperature at 3,000 m, T₁: 6.0ºC
- Pressure at 3,000m, P₁: 0.565atm
At ground:
- Temperature at ground, T₂: 20ºC
- Pressure at ground, P₂: 765mmHg
- Volume at ground, V₂: 30.0 liters
Questions:
- Will the balloon's volume be more than 47.0 liter?
- Amount in grams of helium in the ballon:?
2. Unit conversions:
a) Convert P₂ to atm:
- P₂ = 765mmHg × 1 atm/760.0mmHg = 1.00657895atm
b) Convert the temperatures to kelvin:
- T₁ = 6.0 + 273.15 = 279.15K
- T₂ = 20 + 273.15 = 293.15K
3. Solution
Question 1.
Calculate the final volume of the balloon:
Since the number of moles of helium gas inside the balloon remains unchanged, pV = nRT yields the combined gas law:
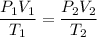
From which you can solve for V₁ and compute it:
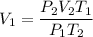
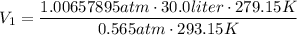
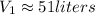
Then, the volume of the balloon will exceed the gurantee of the manufacturer.
Question 2.
a) Calculate the number of moles of helium
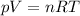
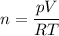
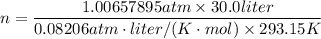
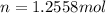
b) Calculate the mass in grams of helium
Use the atomic mass of helium: 4.003g/mol
- mass = number of moles × molar mass
- mass = 1.2558mol × 4.003g/mol = 5.02g
Rounding to 2 significant figures: 5.0g