Answer:
6 moles of Calcium chloride will produce 2 moles of Calcium phosphate.
Step-by-step explanation:
The reaction for the equation is:
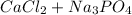 →
→
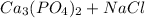
In order to carry determine the stoichometry of this reaction, we need to first balance the equation, to determine amount amounts in moles of both reactants and products.
The balanced equation for this reaction will then be:
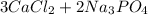 →
→
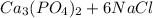
Mole ratio of calcium chloride to calcium phosphate is 3 : 1
which means 3 moles of CaCl2 produced 1 mole of Ca3(PO4)2.
⇒ 6 moles of CaCl2 would produce 2 moles of Ca3(PO4)2