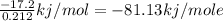Answer:
Enthalpy Change when 1 mole CaCl₂ dissolves
= - 81.13 kj/mole
Step-by-step explanation:
Given information
Mass of CaCl₂ = 23.6 g
Temperature changes from 25.0°C to 38.7°C
Heat capacity of solution & calorimeter (C) = 1258 J/°C
We know Q = C x ΔT
⇒ Heat released = 1258 x (38.7 - 25)
= 1.7234 x 10⁴ J
= 17.2 Kilo-joule
Thus 23.6 g is equivalent to 1.7234 x 10⁴ J or 17.2 Kilo-joule of heat energy
Molar mass of calcium chloride (110.98)
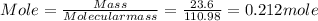
Enthalpy Change when 1 mole CaCl₂ dissolves
=