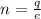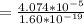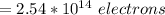Complete Question
The current supplied by a battery as a function of time is I(t) = (0.88 A) e^(-(t * 6 hr)). What is the total number of electrons transported from the positive electrode to the negative electrode from the time the battery is first used until it is essentially dead?
Answer:
The total number of electrons transported is
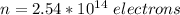
Step-by-step explanation:
Charge is generally expressed as
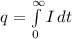
Where I is the current and it is given as
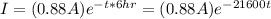
Substituting into equation above d
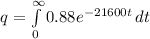
![q = 0.88[-(e^(-21600t))/(21600) ]\left \ {{\infty} \atop {0}} \right.](https://img.qammunity.org/2021/formulas/physics/college/lxk8iu57r60iuaqjf7w7bbo5vydqh1ld62.png)
![q = - (0.88)/(21600) [-1]](https://img.qammunity.org/2021/formulas/physics/college/8fommz1vac6xm2atx3ck9dv6t913ja8rhx.png)
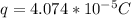
This charge can also be expressed as
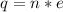
Where is the number of electron
Making n the subject